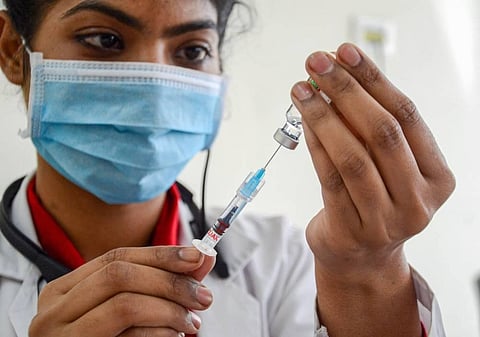

HYDERABAD: Another Hyderabad-based pharmaceutical company developing a Covid-19 vaccine, Biological E Limited, has received the approval to conduct Phase-III clinical trials for its vaccine from the Central Drugs Standard Control Organisation’s (CDSCO) Subject Expert Committee.
This was announced in a press release by the company on Friday The company completed the Phase I/ II clinical trials of its Covid-19 subunit vaccine candidate in India, which began in the second week of November, 2020. The trials were conducted on about 360 healthy subjects in the age group of 18- 65 years.