
As COVID-19 continues to ravage countries across the world and take a heavy toll, people are praying frantically for a targeted treatment or vaccine. Though a specific panacea for the disease still seems a far cry, there is a ray of hope.
Doctors and scientists have now found some tentative evidence that those who are worst hit by SARS CoV 2 can benefit from the infusion of blood plasma collected from others who have recovered from the infection.
There has been limited anecdotal evidence on this from China, US and a few other countries and clinical trials have now begun in some parts to assess how effective the method is against the infection.
Here, we explain the principle behind the therapy and how it could be put into practice in the weeks ahead as the fight against the pandemic continues.
What is plasma therapy?
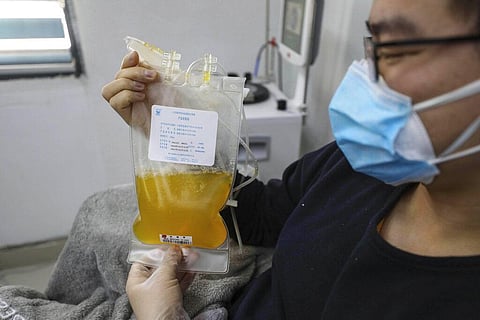
Convalescent plasma therapy involves transfusing specific components from the blood of people who have recovered from COVID-19 -- at least 14 days after their complete recovery -- into people who are very ill with the infection or even people who are at high risk of contracting the virus.
How does the therapy work?
As patients’s bodies grapple with SARS CoV 2, they produce antibodies to attack the virus. Those antibodies -- proteins that are secreted by immune cells called B lymphocytes -- are found in plasma or the liquid part of blood that helps it clot when needed and supports immunity.
Once a person has had the virus and recovered, they have these antibodies or "memory cells" that will stay in their blood waiting to fight the same virus in case there is recurrence of infection. These antibodies, when injected into another person with the disease, recognize the virus as “enemies” and kill it.
In the case of the novel coronavirus, scientists estimate that antibodies attack the spikes on the outside of the virus, blocking the virus from penetrating human cells and replicating.
WATCH | What is hydroxychloroquine and why is India giving it to the US?
Who can benefit from the therapy?
Scientists are estimating that convalescent plasma can be effective in treating people with the most severe symptoms of the virus. It is also being hoped that the therapy could prevent the condition of those moderately sick with the infection from deteriorating.
Is there a limitation to the therapy?
Convalescent plasma is also known as passive antibody therapy, meaning that while it can immediately provide a person with antibodies to fight a virus, those antibodies only last a short period of time in the recipient’s body and therefore the hope is pinned on the body’s own ability to develop its own defence against the virus. This is unlike the donor's case where the antibodies are long-term. There is no conclusive evidence yet but some virologists have said that once infected and recovered, COVID-19 patients will never get the disease again.
How many patients can be helped by one plasma donor?
So far, it has been seen that one person’s donation of plasma can produce two doses of blood component needed for transfusions. It has also been estimated that a person only needs one transfusion to get enough antibodies to fight the virus.
Has plasma therapy been tried on COVID-19 patients in India?
No. The treatment protocol for very sick COVID-19 patients in India includes a combination of anti-malaria drug Hydroxycholoroquine and antibiotic Azithromycin as of now. The 21-member task force on managing the COVID-19 outbreak, however, is considering to direct the Indian Council of Medical Research on coming up with a protocol for introducing the therapy in India in severe patients.