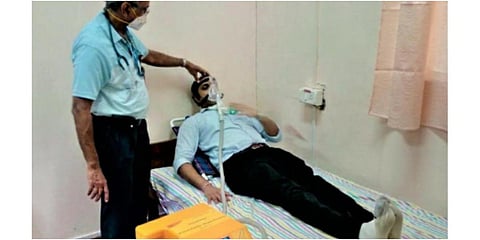

BENGALURU: The National Aerospace Laboratories (NAL) on Thursday said it would soon start human clinical trial of its indigenously developed non-invasive ventilator "SwasthVayu" to treat COVID-19 patients.
The trial of the first Made in India non-invasive ventilator would begin at the Manipal Hospital, which also collaborated in its development in 'record' 36 days, the NAL said in a statement here.
The device can be used for COVID patients and also support those suffering from other respiratory disorders and heart failure, it said.
NAL had in May last announced the development of the ventilator which was simple to use without any specialised nursing and cost effective, compact and configured with majority of indigenous components.
Since then it had been tested on artificial lung models with successful results, the institute said.
SwastVayu was developed by the NAL, a part of the Council of Scientific and Industrial Research (CSIR), in scientific and medical knowledge collaboration with Dr Satyanarayana Mysore, head of the Pulmonology Respiratory and Sleep Medicine at Manipal Hospital, and Dr Anurag Agarwal, Director of CSIR-Institute of Genomics and Integrative Biology (IGIB).
"The Ethics Committee, and the scientific committee at Manipal Hospital, Bengaluru has scrutinized and approved the device for clinical trials under Dr Satyanarayana, Head of Pulmonology and Sleep Medicine as the Principal Investigator," the statement said.
It added that there was a compelling need for indigenous ventilators to mitigate the acute shortage of ventilators.
The statement quoted Dr Satyanarayana as saying that the device will also be a bonanza post-pandemic for treating sleep-disordered breathing including obstructive sleep apnea and other sleep apnea.
The clinical trial will begin shortly and for now, the focus will be limited to its successful completion, he said.
Chief scientist and Head Electronics Division of NAL Dr C M Ananda said the device had been subjected to trials on artificial lung models and has successfully passed stringent electrical safety, performance, calibration, bio-compatibility tests at National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited laboratory.
According to the NAL, SwasthVayu is equipped with advanced features like Bi-level mode (BiPAP), Continuous Positive Airway Mode (CPAP), Spontaneous modes and 3D printed HEPA-T filter adapter connected directly to the non-ventilated mask.