Hyderabad startup reverses chronic liver failure in animal trials
HYDERABAD: A Hyderabad-based startup, Tulsi Therapeutics, has demonstrated the complete reversal of chronic liver failure in animal trials using an innovative stem cell-exosome therapy.
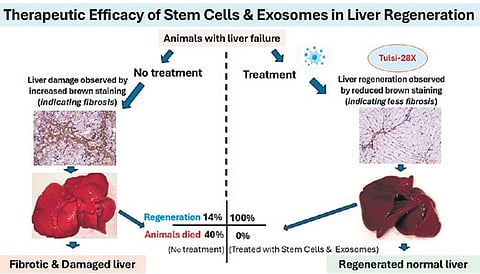
Conducted on rats, the preclinical study showed 100% liver fibrosis reversal and zero mortality among treated subjects, in stark contrast to the untreated group that saw only 14% reversal and 43% deaths.
Tulsi-28X is a first-in-class regenerative biologic derived from Wharton’s Jelly mesenchymal stem cells and their native exosomes, a combination never before tested in any animal model globally. Tulsi Therapeutics, incubated at ASPIRE-BioNEST, University of Hyderabad, developed the platform entirely in India over three years.
Chronic liver failure accounts for nearly 20% of all liver-related deaths globally, with liver transplantation being the only treatment. Founder and CEO of Tulsi Therapeutics Dr Sairam Atluri said, “There have been individual successes with stem cells and exosomes, but we combined them because they operate through different mechanisms. This combination maximised the biological outcome and marks a major milestone for India’s biotech sector.”
Looking ahead, the chief scientific officer said the next goal is to initiate human clinical trials in partnership with Nizam’s Institute of Medical Sciences (NIMS), with groundwork expected to take up to two years.
The results were presented at the AASLD 2024 Liver Conference in San Diego and have also been accepted for publication in the Journal of Regenerative Medicine.